Moderna Files for Authorization of Its COVID-19
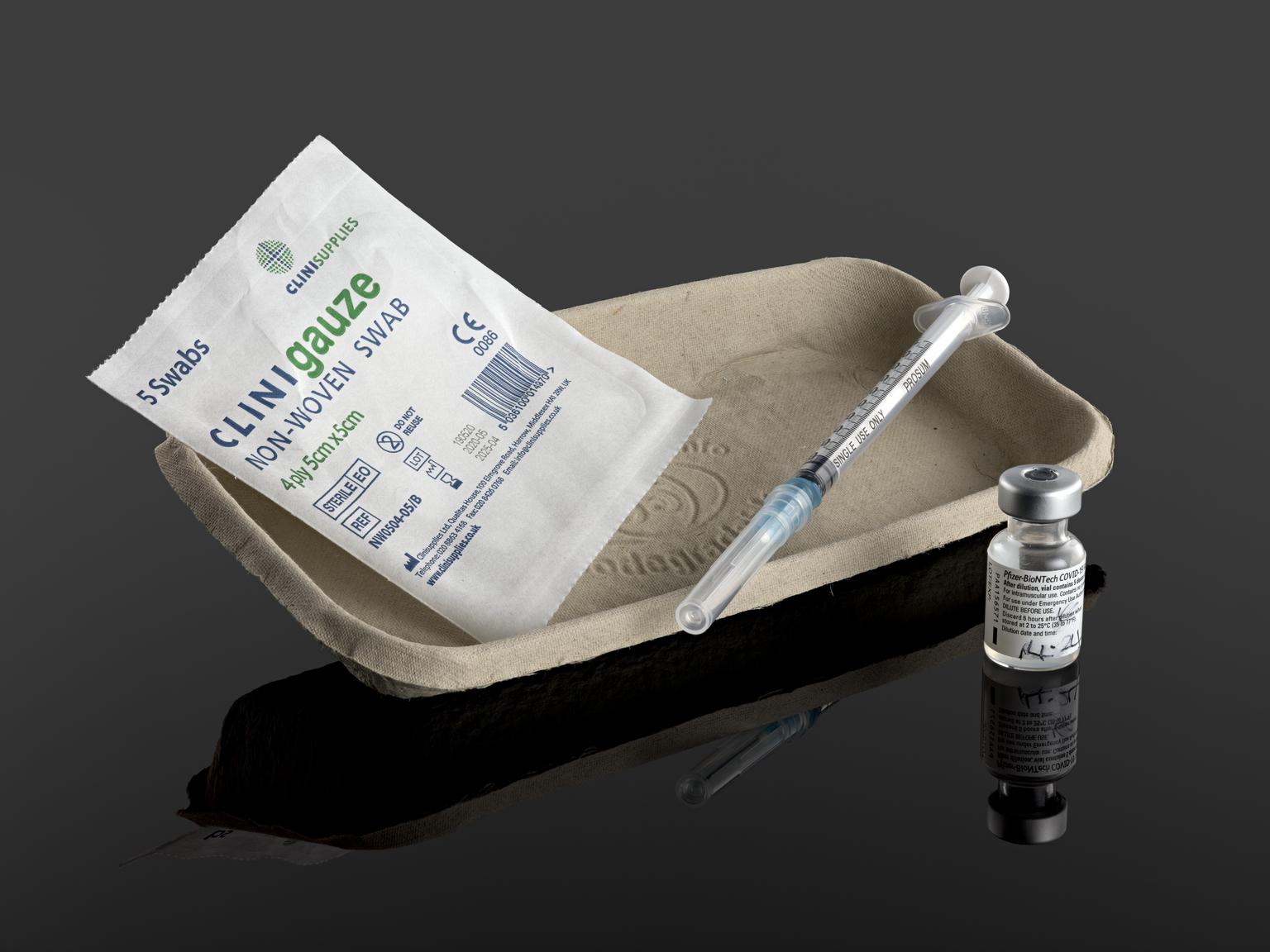
Vaccine in Young Children Six Months to Under Six Years of Age Submission to Regulators Globally Is Based on Phase 2/3 Studies of mRNA-1273 in Young Children CAMBRIDGE, MA / ACCESSWIRE / Moderna, Inc. (NASDAQ:MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that it has submitted a request for emergency […]
FDA Issues Decisions on Additional E-Cigarette Products

Agency Permits Marketing of Certain Tobacco-Flavored Products, Denies Other Products; Additional Decisions on Popular Products Expected Soon Today, the U.S. Food and Drug Administration took additional actions as part of the agency’s work to ensure any electronic nicotine delivery system (ENDS) products available for sale have demonstrated that marketing of the products is appropriate for […]
FDA Approves Treatment for Wider Range of Patients with Heart Failure

Today, the U.S. Food and Drug Administration approved Jardiance (empagliflozin) to reduce the risk of cardiovascular death and hospitalization for heart failure in adults. Jardiance was originally approved by the FDA in 2014 as a supplement to diet and exercise to improve glucose control in adults with type 2 diabetes. Jardiance is also approved to […]
FDA Approval Of Kerendia To Help Slow Kidney Disease And Failure Associated With Type 2 Diabetes

New Kidney Drug Therapy and the FDA The American Association of Kidney Patients, the largest kidney patient consumer and caregiver organization in the nation, today issued the following statement regarding the recent approval by the U.S. Food and Drug Administration of a new drug therapy, Kerendia by Bayer Pharmaceuticals, designed to slow chronic kidney disease […]
FDA Approves Component of Treatment Regimen for Most Common Childhood Cancer
Alternative Has Been in Global Shortage Since 2016 The U.S. Food and Drug Administration approved Rylaze (asparaginase erwinia chrysanthemi (recombinant)-rywn) as a component of a chemotherapy regimen to treat acute lymphoblastic leukemia and lymphoblastic lymphoma in adult and pediatric patients who are allergic to the E. coli-derived asparaginase products used most commonly for treatment. The […]
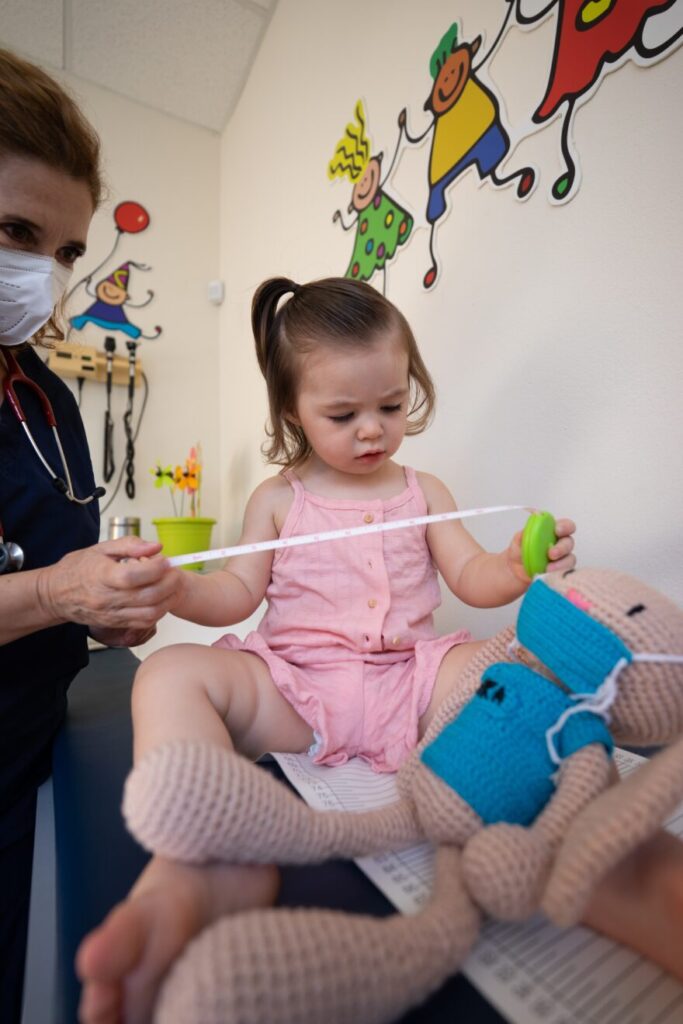
FDA Approves First Oral Blood Thinner for Children
Today, the U.S. Food and Drug Administration approved Pradaxa (dabigatran etexilate) oral pellets to treat children 3 months to less than 12 years old with venous thromboembolism (a condition where blood clots form in the veins) directly after they have been treated with a blood thinner given by injection for at least five days. The […]
FDA Approves a Nasal Antihistamine for Nonprescription Use

Approval is a First-in-class and was Enabled by the Prescription to Nonprescription Switch Process The U.S. Food and Drug Administration today approved a nasal antihistamine for nonprescription use through a process called a partial prescription to nonprescription switch. The FDA approved Astepro (azelastine hydrochloride nasal spray, 0.15%) for seasonal and perennial allergic rhinitis—commonly known as […]
FDA Launches Challenge to Spur Development of Affordable Traceability Tools as Part of Broader Food Safety Efforts

Affordable Traceability Tools The U.S. Food and Drug Administration launched a challenge to spur the development of affordable, tech-enabled traceability tools to help protect people and animals from contaminated foods by enabling the rapid identification of their sources and helping remove them from the marketplace as quickly as possible. The FDA New Era of Smarter […]
FDA Seeks Further Investments in Critical Public Health Infrastructure and Medical Product Safety Programs

Fiscal Year 2022 Budget Request Reflects Nearly 8% Increase from Previous Year and Focuses on Agency’s Immediate Priorities The U.S. Food and Drug Administration is requesting a total budget of $6.5 billion as part of the President’s fiscal year (FY) 2022 budget – a nearly 8% ($477 million) increase over the agency’s FY 2021 […]
FDA Approves First Targeted Therapy for Previously Resistant Lung Cancer Mutation

Today, the U.S. Food and Drug Administration approved Lumakras (sotorasib) as the first treatment for adult patients with non-small cell lung cancer whose tumors have a specific type of genetic mutation called KRAS G12C and who have received at least one prior systemic therapy. This is the first approved targeted therapy for tumors with any […]


