What You Need to Know about Omicron

Omicron in the United States CDC is working with state and local public health officials to monitor the spread of Omicron. As of December 20, 2021, Omicron had been detected in most states and territories and continues to be the dominant variant in the United States. What We Know about Omicron CDC has been collaborating with […]
A potential at-home COVID-19 test is just as good as laboratory PCR tests, according to preclinical data
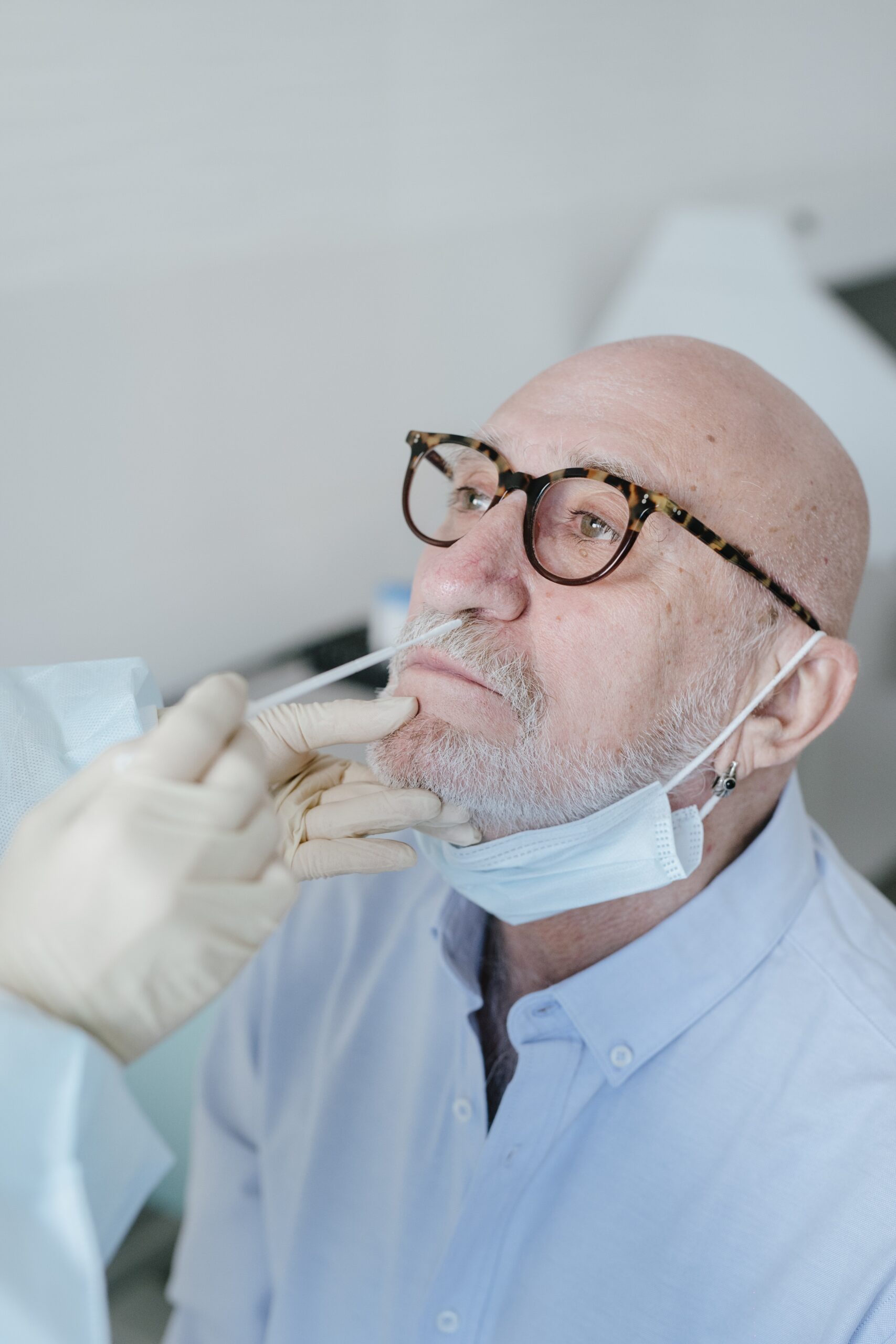
More research needed to validate test Researchers at the University of Illinois Chicago have designed a high-quality assay that can be used in at-home tests for rapid COVID-19 screening. Results from an early preclinical study suggest that tests with the new assay may be just as reliable as the laboratory-based molecular tests — called PCR tests — used by hospitals […]
NASA Commits $28 Million to Underfunded US Jurisdictions

NASA has awarded $28 million to fund the next five years of research infrastructure development across 28 jurisdictions. The Established Program to Stimulate Competitive Research (EPSCoR), a part of NASA’s Office of Stem Engagement and based out of the agency’s Kennedy Space Center in Florida, supports science and technology research and development at colleges and […]
ACT−Accelerator partnership welcomes leadership and commitments at US COVID Summit to ending COVID−19 pandemic through equitable access to tests, treatments, and vaccines
President Biden and global leaders agreed ambitious targets, aligned with the ACT-Accelerator, to end the COVID-19 pandemic Collective accountability for world leaders, industry and partners emphasized, as global response now turns to implementation of these targets Concrete action must now follow this summit to deliver and deploy tests, treatments, and vaccines immediately, to prevent further […]
Phoenix City Council Approves Public Transit Resources for the Most Vulnerable

The Phoenix city council voted unanimously today to approve $1 million to help residents in need with the cost of riding public transit. The city will use federal American Rescue Plan Act (ARPA) funds to purchase more than 31,000 monthly transit passes. The move significantly increases the number of reduced-fare passes for those passengers […]
FDA Approves First COVID-19 Vaccine

Today, the U.S. Food and Drug Administration approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization […]
Rigel Pharmaceuticals Provides Update on COVID-19 Program

SOUTH SAN FRANCISCO, Calif. — Rigel Pharmaceuticals, Inc. (Nasdaq: RIGL), today announced that the U.S. Food and Drug Administration (FDA) has informed the Company that clinical data submitted in late-May from a 59-patient NIH/NHLBI-sponsored Phase 2 trial of fostamatinib to treat hospitalized patients suffering from COVID-19 are insufficient for an emergency use authorization (EUA) at this time. […]
Covid 19 – What Should I Do?

Covid 19 – What Should I Do? By George Uliano It has been over a year since the Covid19 Pandemic struck the world. What should you do now? First, I am not a Doctor, I do not even have a background in the medical field. My background is in the security and lock business. […]
Patients hospitalized for COVID-19 in 2021 could pay thousands of dollars, study suggests

Newswise — Americans who get seriously ill from COVID-19 in 2021 might have to pay thousands of dollars in bills from their hospitals, doctors and ambulance companies, a new study suggests. The new University of Michigan analysis has implications for both policymakers and people who haven’t yet gotten vaccinated. The authors published their findings as […]



